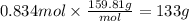Answer:
133 g
Step-by-step explanation:
Step 1: Write the balanced equation
2 Al(s) + 3 Br₂(l) ⇒ 2 AlBr₃(s)
Step 2: Calculate the moles corresponding to 15.0 g of Al
The molar mass of aluminum is 26.98 g/mol. The moles corresponding to 15.0 g of Al are:
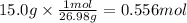
Step 3: Calculate the moles of Br₂ that react with 0.556 moles of Al
The molar ratio of Al to Br₂ is 2:3. The moles of bromine that react with 0.556 moles of aluminum are:
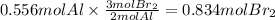
Step 4: Calculate the mass corresponding to 0.834 moles of Br₂
The molar mass of bromine is 159.81 g/mol. The mass corresponding to 0.834 moles of Br₂ is: