Answer:
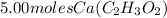
Step-by-step explanation:
First find the chemical formula for this reaction:
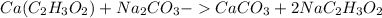
Knowing from the chemical formula that one mole of calium acetate will react with one mole of sodium carbonate can be used to find how many moles of calcium acetate react with 5.00 moles of sodium carbonate:
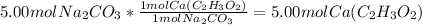
Remember that your answer needs to have three significant figures.