Answer:
The electron spin quantum number is
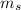 and does not depend on another quantum number. It is +1/2 (↑) and -1/2 (↓). For the sulfur, the spin is -1/2.
and does not depend on another quantum number. It is +1/2 (↑) and -1/2 (↓). For the sulfur, the spin is -1/2.
Step-by-step explanation:
The quantum numbers for atoms are:
- n: the principal quantum number = 1, 2, 3, 4, 5, 6
- l: the orbital angular momentum quantum number = 0, 1, 2, 3, ... n-1
-
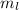 : the magnetic quantum number = -l, .. -1, 0, 1, .. l
: the magnetic quantum number = -l, .. -1, 0, 1, .. l
-
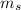 : the electron spin quantum number = +1/2 (↑), -1/2 (↓). This quantum number does not depend on another quantum number.
: the electron spin quantum number = +1/2 (↑), -1/2 (↓). This quantum number does not depend on another quantum number.
The sulfur, Z = 16 has the following electronic configuration:
1s² 2s² 2p⁶ 3s² 3p⁴ → [Ne] 3s² 3p⁴
Let's now see the quantum numbers for the orbital 3p⁴ (the last electron filling orbital) for the sulfur:
- n = 3
- l = 1 (since 0 = s, 1 = p, 2 = d, 3 = f)
-
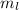 = -1, since the last electron filled in the orbital p (the fourth electron) is in ml = -1:
= -1, since the last electron filled in the orbital p (the fourth electron) is in ml = -1:
↑↓ ↑ ↑
n = 3
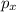
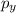
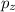
ml = -1 0 +1
-
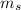 = -1/2. This is because the last electron filled is in ml = -1, in a down position ↓.
= -1/2. This is because the last electron filled is in ml = -1, in a down position ↓.
Therefore, the electron spin quantum number is
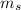 , and does not depend on another quantum number. It is +1/2 when the electron is ↑ and -1/2 when the electron is ↓. Thus, for the sulfur the last electron filled in the orbital is ↓, so the spin is -1/2.
, and does not depend on another quantum number. It is +1/2 when the electron is ↑ and -1/2 when the electron is ↓. Thus, for the sulfur the last electron filled in the orbital is ↓, so the spin is -1/2.
I hope it helps you!