Answer:
3.24
Step-by-step explanation:
It is known that nitric acid is a strong acid, so it will dissociate completely, resulting in
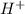 and
and
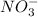 ions being present in solution. We also know that pure water has a neutral pH due to the chemical equilibrium of
ions being present in solution. We also know that pure water has a neutral pH due to the chemical equilibrium of
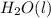 ⇄
⇄
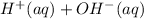 signifying equal parts of hydrogen and hydroxide ions.
signifying equal parts of hydrogen and hydroxide ions.
Now, we must convert from 4.5 moles of nitric acid to moles of hydrogen. This can be accomplished using unit conversions and the chemical dissociation equation
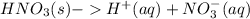
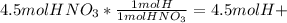
Then, using the pH equation
![-log[H+] = pH](https://img.qammunity.org/2023/formulas/chemistry/high-school/hq4zae88whbvp7rpsh8n18uqis7rbql49h.png) and the formula for molarity
and the formula for molarity
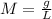 , we can find the value of
, we can find the value of
![[H^+]](https://img.qammunity.org/2023/formulas/chemistry/high-school/15tcfhlf0www29xcj3ecoj02pw3y7uttb5.png) and in turn the pH of the resulting solution. Keep in mind that both answers for grams of the hydrogen ion and molarity of the hydrogenion should have two significant figures.
and in turn the pH of the resulting solution. Keep in mind that both answers for grams of the hydrogen ion and molarity of the hydrogenion should have two significant figures.
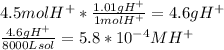
Now the pH equation may be used.
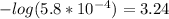
Note that logarithmic significant figures dictate that the logarithm of a number with x significant figures will result in a value with x digits after the decimal point, meaning that this answer will have two digits after the decimal point and three significant figures.