Answer:
B) 10.1 L
Step-by-step explanation:
Hello,
In this case, for the given chemical reaction which should be corrected as shown below:
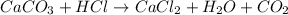
Since 45.0g of calcium carbonate are used, the produced moles of carbon dioxide, via stoichiometry, are found to be:
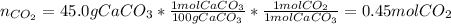
Finally, since STP conditions are referred to a temperature of 273.15K and 1 atm, the volume, by using the ideal gas equation result:
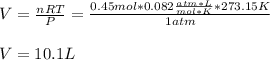
So the answer is B) 10.1 L.
Best regards.