Answer:
B
Step-by-step explanation:
We can use Planck's equation. Recall that:
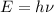
Where h is Planck's constant and ν is the frequency.
We also have that:
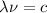
Where c is the speed of light and λ is the wavelength. Hence:
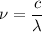
Therefore:
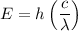
Solving for λ yields:
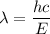
Hence substitute. Recall that h = 6.626 × 10⁻³⁴ Js and c = 2.998 × 10⁸ m/s:
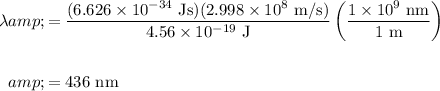
In conclusion, our answer is B.