Answer:
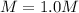
Step-by-step explanation:
Hello,
In this case, since the molarity is defined as the ratio regarding the moles of solute to the volume of the solution in liters, for such ionic salts like potassium iodide, the volume of the solution does not significantly changes once the solution is prepared, for that reason, the resulting molarity is:
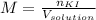
However, the moles of potassium iodide turn out:
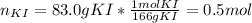
And the volume of the solution:
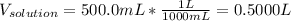
Finally, the molarity results:
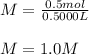
Best regards.