Answer:
1.48×10²⁴ Cu atoms
Step-by-step explanation:
For this question you need to use Avogadro's number 6.022×10²³atoms.
2.45 moles of Cu ×
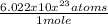 = 1.47539×10²⁴ atoms.
= 1.47539×10²⁴ atoms.
The moles cancel out so you are left with atoms.
Since there are 3 significant figures in the question there should be 3 significant figures in your answer, which is 1.48×10²⁴ Cu atoms.