Answer:
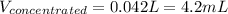
Step-by-step explanation:
Hello,
In this case, since in a dilution process the moles of the solute must remain unchanged, we use the volumes and molarities as shown below:
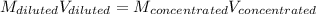
Clearly, the concentrated solution is 12M and the diluted solution is 0.5 M, thus, the volume of the concentrated solution we should take is:
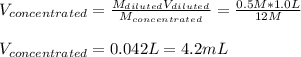
Best regards.