Answer:
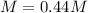
Step-by-step explanation:
Hello,
In this case, the molarity is defined as the ratio of the moles of the solute to the volume of the solution in liters:
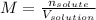
In this case, the solute is the KCl (potassium chloride) and the solution is made up of both water and KCl. Moreover, since during this type of dissolution processes, the volume of the solution is not significantly affected by the addition of the solute, the resulting molarity is:
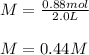
Best regards.