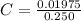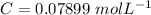Answer:
The concentration of KBr is
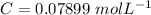
Step-by-step explanation:
From the question we are told that
The mass of KBr is
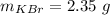
The molar mass of KBr is
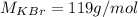
Volume of water is
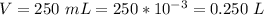
This implies that the volume of the solution is
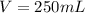
The number of moles of KBr is
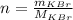
Substituting values
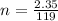
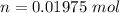
The concentration of KBr is mathematically represented as