Answer:
The change in temperature is 77.51 K.
Step-by-step explanation:
We have,
Mass of gold, m = 100 g
Heat supplied, Q = 1000 J
It is required to find the temperature change when 100 block of gold is supplied with 1000 J of heat.
The specific heat of gold is
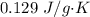 . The heat supplied of released is given by :
. The heat supplied of released is given by :
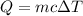
 is change in temperature
is change in temperature
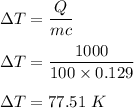
So, the change in temperature is 77.51 K.