Answer:
The amount of heat released is -5,232.5 J
Step-by-step explanation:
Calorimetry is the measurement and calculation of the amounts of heat exchanged by a body or a system.
The amount of heat received or transferred by a body when it undergoes a temperature variation (Δt) without there being a change of physical state (solid, liquid or gaseous) is calculated by the expression:
Q = c * m * ΔT
Where Q is the heat exchanged by a body of mass m, made up of a specific heat substance c and where ΔT is the temperature variation.
In this case:
- c=4.186
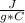
- m= 250 g
- Δ=Tfinal - Tinitial= 25°C - 30°C= -5 °C
Replacing:
Q= 4.186
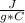 *250 g* (-5°C)
*250 g* (-5°C)
Solvng:
Q= -5,232.5 J
The amount of heat released is -5,232.5 J