Answer:
26.3 moles of O₂ are needed to react completely with 35.0 mol of FeCl₃
Step-by-step explanation:
To determine the number of moles of O₂ that are needed to react completely with 35.0 mol of FeCl₃, it is possible to use the reaction stoichiometry of the reaction (that is, the relationship between the amount of reagents and products in a chemical reaction), and rule of three as follows: if 4 moles of FeCl₃ react with 3 moles of O₂, 35 moles of FeCl₃ with how many moles of O₂ will it react?
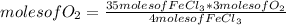
moles of O₂= 26.25 ≅ 26.3
26.3 moles of O₂ are needed to react completely with 35.0 mol of FeCl₃