Hello There!!
We are given to find efficiency of the cycle.
We Know
Efficiency is denoted by the symbol "η"
![\text{η\%} = \frac{ \text{Output Work}}{ \text{Heat Supplied } } * 100]()
As it is a cyclic process, So,
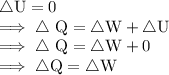
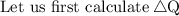
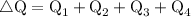
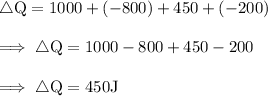
![\therefore \text{ Net Output Work i.e., ∆W = 450 J}]()
Now, We need to find the Heat Input.
We know, Heat Input is always the sum of all positive heats
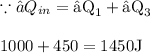
Now We Have To Find The η%
![\text{η\%} = \frac{ \text{Output Work}}{ \text{Heat Supplied } } * 100]()
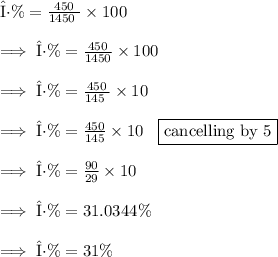
Option A= 31% is the correct answer
Hope this helps!!