Answer:
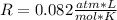
Step-by-step explanation:
Hello,
In this case, by knowing that hydrogen gas could be considered as an ideal gas, the formula we use is:

In which we are asked to compute the ideal gas constant R by knowing that the temperature must be used in absolute units (Kelvins), thus we obtain:
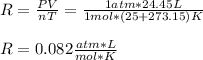
Best regards.