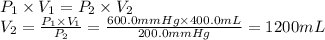Answer:
1200 mL
Step-by-step explanation:
Given data
- Initial pressure (P₁): 600.0 mmHg
- Initial volume (V₁): 400.0 mL
- Final pressure (P₂): 200.0 mmHg
For a gaseous sample, there is an inverse relationship between the pressure and the volume. If we consider the gas as an ideal gas, we can find the final volume using Boyle's law.