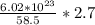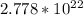Answer:
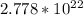
Step-by-step explanation:
Weight of NaCl is equal to sum of weight of one mole of sodium and one mole of chloride.
Mathematically, it can be represented as -
1 mol (Na)
 1 mol (Cl)
1 mol (Cl)
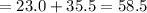 g/mol
g/mol
In
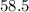 gram of NaCl, there is one mole of Na or
gram of NaCl, there is one mole of Na or
 ions
ions
In one gram of NaCl, there will be
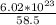 sodium ions
sodium ions
In
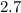 grams of NaCl, the number of sodium ions will be -
grams of NaCl, the number of sodium ions will be -