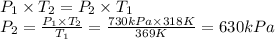Answer:
630 kPa
Step-by-step explanation:
Given data
- Initial pressure (P₁): 730 kPa
- Initial temperature (T₁): 96 °C
- Final temperature (T₂): 45 °C
Step 1: Convert the temperatures to the absolute scale (Kelvin)
When working with gases, we always need to convert the temperatures to the Kelvin scale. We will use the following expression.
K = °C + 273.15
T₁: K = 96 °C + 273.15 = 369 K
T₂ = K = 45 °C + 273.15 = 318 K
Step 2: Calculate the final pressure
We will use Gay-Lussac's law.