Answer:
6 moles of oxygen are needed to make 12 moles of magnesium oxide.
Step-by-step explanation:
First of all you should know that the balanced chemical equation is:
2 Mg + O₂ → 2 MgO
The rule of three or is a way of solving problems of proportionality between three known values and an unknown value, establishing a relationship of proportionality between all of them. That is, what is intended with it is to find the fourth term of a proportion knowing the other three. Remember that proportionality is a constant relationship or ratio between different magnitudes.
If the relationship between the magnitudes is direct, that is, when one magnitude increases, so does the other (or when one magnitude decreases, so does the other) , the direct rule of three must be applied. To solve a direct rule of three, the following formula must be followed:
a ⇒ b
c ⇒ x
So:
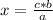
In this case the rule of three applies as follows: if 2 moles of magnesium oxide are produced from 1 mole of oxygen, 12 moles of magnesium oxide from how many moles of oxygen is produced?
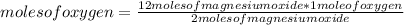
moles of oxygen= 6
6 moles of oxygen are needed to make 12 moles of magnesium oxide.