Answer:
The volume of carbon (ii) oxide present is 2.80 L
Step-by-step explanation:
In this question, we are asked to calculate the volume of a certain number of moles of carbon (ii) oxide sealed in a container at STP
The equation to use here is the ideal gas equation.
Mathematically;
PV = nRT
since we are to calculate the volume, the equation can be re-written as
V = nRT/P
The values according to the question are as follows;
n is the number of moles which is 0.125 moles
T is the temperature at STP which is 0°C = 273.15 K(kelvin)
P is the pressure at STP which is 1.0 atm (atmosphere)
R is the molar gas constant which is 0.0821 L.atm.
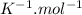
Plugging these values, we have
V = (0.125 × 0.0821 × 273.15)/1 = 2.80 L