Answer:
Step-by-step explanation:
mole of O₂ =
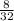
= .25 moles
mole of CO₂
=
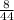
= .1818 moles
moles of SO₂
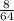
= .125 moles
Total moles of gas
= .5568 moles.
total volume of gas mixture
= 22.4 x .5568 liter ( volume of one mole of any gas = 22.4 liter)
= 12.47 liter.
gas will exert partial pressure according to their mole fraction
gas having greatest no of moles in the total mole will have greatest mole fraction so
O₂ will have greatest partial pressure.