Answer:
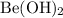 and
and
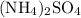 . The missing ion would be
. The missing ion would be
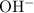 .
.
Step-by-step explanation:
In a double replacement reaction, two ionic compounds exchange their ions to produce two different ionic compounds.
In this question, the two ionic compounds are:
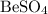 , and
, and
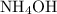 .
.
In particular,
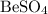 is made up of
is made up of
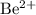 ions and
ions and
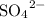 ions, while
ions, while
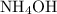 is made up of
is made up of
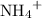 ions and
ions and
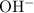 ions.
ions.
In a binary ionic compound, cations (positive ions) can only bond to anions (negative ions.)
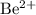 is a cation. In
is a cation. In
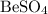 ,
,
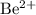 was bounded
was bounded
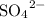 anions. During the reaction, it bonds with
anions. During the reaction, it bonds with
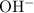 anions to produce
anions to produce
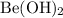 .
.
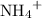 is also a cation. In
is also a cation. In
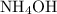 ,
,
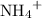 was bounded to
was bounded to
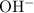 ions. During the reaction, it bonds with
ions. During the reaction, it bonds with
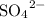 anions to produce
anions to produce
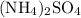 .
.
Hence, the two products will be
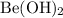 and
and
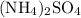 .
.
Note that charges on the ions must balance. For example, a
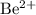 ion carries twice as much charge as an
ion carries twice as much charge as an
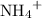 ion. As a result, each
ion. As a result, each
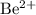 ion would bond with twice as many
ion would bond with twice as many
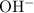 ions as
ions as
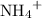 would in
would in
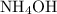 .
.