Answer:
M = 0.177
Step-by-step explanation:
First, we have to find the molar mass of potassium iodine (KI).
K = 39.098
I = 126.904
Now, add these values together to get the molar mass of KI
39.098 + 126.904 = 166.0028
Now, it's time to do a grams to moles conversion.
35.0 g KI *
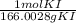 =
=
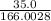 = 0.212 mol KI
= 0.212 mol KI
Now, we can find the molarity of this solution.
Molarity (M) =
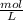
M =
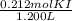 = 0.177 M
= 0.177 M
The molarity (M) of this solution is 0.177.