Answer:
Final pressure in (atm) (P1) = 6.642 atm
Step-by-step explanation:
Given:
Initial volume of gas (V) = 12.5 L
Pressure (P) = 784 torr
Temperature (T) = 295 K
Final volume (V1) = 2.04 L
Final temperature (T1) = 310 K
Find:
Final pressure in (atm) (P1) = ?
Computation:
According to combine gas law method:
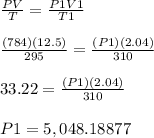
⇒ Final pressure (P1) = 5,048.18877 torr
⇒ Final pressure in (atm) (P1) = 5,048.18877 torr / 760
⇒ Final pressure in (atm) (P1) = 6.642 atm