Answer:
The correct answer to the following question will be "62.9 %".
Step-by-step explanation:
The given values are:
The aspirin's initial amount = 5.945 g.
and is polluted containing 2,134 g of sodium sulfate.
After extraction we provided 3,739 g of pure aspirin.
Now,
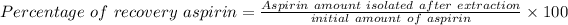
On putting the values in the above formula, we get
⇒
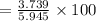
⇒
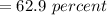
Note: percent = %