Answer:
Step-by-step explanation:
Zn + 2HCl = ZnCl₂ + H₂
A ) mole of Zn = 2.65 / 65
= .04 mol
mole of HCl required = .04 x 2 mol
.08 mol
If v be the volume required
v x 6 = .08
v = .0133 liter
= 13.3 cc
B )
volume of gas at NTP :
moles of gas obtained = .04 moles
= 22.4 x .04 liter
= .896 liter
we have to find this volume at given temperature and pressure
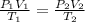
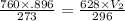
 = 1.175 liter.
= 1.175 liter.
C )
.04 mole of zinc chloride will be produced
mol weight of zinc chloride
= 65 + 35.5 x 2
= 136 gm
.04 mole = 136 x .04
= 5.44 gm