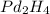Answer: The molecular formula for the given organic compound is
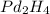
Step-by-step explanation:
The empirical formula for the given compound is
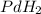
For determining the molecular formula, we need to determine the valency which is multiplied by each element to get the molecular formula.
The equation used to calculate the valency is :
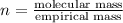
We are given:
Mass of molecular formula = 216.8 g
Mass of empirical formula = 108.4 g
Putting values in above equation, we get:
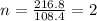
Multiplying this valency by the subscript of every element of empirical formula, we get:
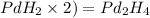
Thus, the molecular formula for the given organic compound is