Answer: The concentrations of the products and reactants do not change.
Step-by-step explanation:
The reaction is given as:
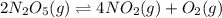
Equilibrium state is the state when reactants and products are present but the concentrations does not change with time.
A chemical equilibrium is dynamic in nature forward and backward reactions continue for indefinite time and never stops. The concentration of the reactants and products remain constant.
Thus when equilibrium is reached, the reaction is still occurring in both directions, but the rates of forward and backward direction are equal so there is no net change in the concentrations of the reactants or products.