Answer:
Step-by-step explanation:
Pressure P = 895 mm of Hg
895/760 atm
= 1.1776 atm
Temperature T = 35 + 273 = 308 K
volume V = 9.55 mL
9.55 x 10⁻³ L
Value of R = .082 Litre-atm/mol.K
Putting the value in gas equation
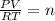 , n is no of moles of gas.
, n is no of moles of gas.
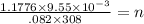
= .4453 x 10⁻³ moles .