Answer:
Approximately 10,5
Step-by-step explanation:
The question is not really very specific, because it would need the percentages of those isotopes in the nature. As they are not shown, it should be the median of those two numbers.
atomic weight ≈
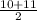 = 10,5
= 10,5
If you check a periodic table, you'll see it's actually 10,8, but that's because of the thing I told you at first (percentages missing).
Hope I could help.