Answer:
The volume occupied by 1 atm of gass is 41.7 L.
Step-by-step explanation:
Given that,
A gas occupies 45.6 L at 695 torr. We need to find the volume occupied by gas if pressure is 1 atm.
For this, firstly it is required to convert torr to atm.
1 atm = 760 torr
It means, we need to find the volume occupied by 760 torr of gas. It is based on Boyle's law. Its mathematical form is given by :
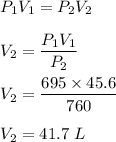
So, volume occupied by 1 atm of gass is 41.7 L.