Answer:
m = 1538.5 g
Step-by-step explanation:
To answer this question, use the equation for specific heat:
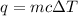
Where q is the total heat energy, m is the mass, c is the specific heat of the substance (in this case water), and delta T is the temperature change.
You will be expected to know the specific heat of water, which is 4.186 J/g/C. Using the information in the question:
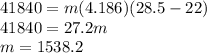
The final answer, 1538.5 g, is closest.