Answer:
See Below.
Step-by-step explanation:
Recall that molarity is defined by moles of solute over liters of solution (mol/L).
Therefore, to make 90.0 mL of 2.0 M MgSO₄, we will need:
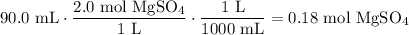
Convert this amount to grams. The molecular weight of MgSO₄ is 120.38 g/mol:
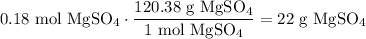
Therefore, to make the solution, we can add 22 grams of MgSO₄ into a graduated cylinder, then mix and dilute the solution with distilled water until we reach 90.0 mL.