Answer:
The temperature is 69.05 K.
Step-by-step explanation:
We have,
Number of moles are 6
Pressure is 3.4 atm and a volume of 10 liters.
It is required to find the temperature. It can be calculated using gas law equation. It says that,

P is pressure
V is volume
n is number of moles
R is gas constant,
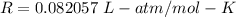
T is temperature
Plugging all the values we get :
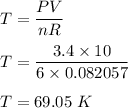
So, the temperature is 69.05 K.