Answer:
- Absolute pressure inside the ball:
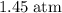 .
. - Number of moles of air particles inside the ball, by the ideal gas law: approximately
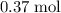 .
.
Step-by-step explanation:
The gauge pressure inside the ball gives the absolute pressure inside the ball, relative to the atmospheric pressure outside the ball. In other words:
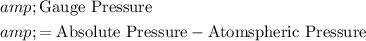 .
.
Rearrange to obtain:
 .
.
Look up the ideal gas constant. This constant comes in a large number of unit combinations. Look for the one that takes
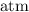 for pressure and
for pressure and
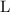 for volume.
for volume.
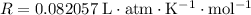 .
.
Convert the temperature to absolute temperature:
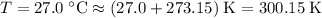 .
.
Assume that the gas inside this ball acts like an ideal gas. Apply the ideal gas law
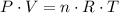 (after rearranging) to find the number of moles of gas particles in this ball:
(after rearranging) to find the number of moles of gas particles in this ball:
 .
.
(Rounded to two significant figures, as in the pressure gauge reading.)