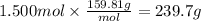Answer:
239.7 g
Step-by-step explanation:
Step 1: Write the balanced equation
2 LiBr + I₂ → 2 LiI + Br₂
Step 2: Convert the molecules of iodine to moles
We have 9.033 × 10²³ particles (molecules) of iodine. In order to convert molecules to moles, we will use the Avogadro's number: there are 6.022 × 10²³ molecules of iodine in 1 mole of iodine.
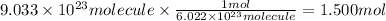
Step 3: Calculate the moles of bromine produced
The molar ratio of I₂ to Br₂ is 1:1. Then, the moles of bromine produced are 1.500 moles.
Step 4: Calculate the mass of bromine
The molar mass of bromine is 159.81 g/mol. The mass corresponding to 1.500 moles is: