Answer:
min = kq^2/5mv^2
Step-by-step explanation:
The total kinetic energy of both electrons will be electrostatic potential energy, when the electrons reach the minima distance due to electrostatic repulsion. Then, you have:
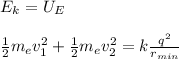
me: mass of the electron
q: charge of the electron
k: Coulomb's constant
you take into account that v2=3v1=3v and do rmin the subject of the formula:
![(1)/(2)m_e[v^2+9v^2]=5m_ev^2=k(q^2)/(r_(min))\\\\r_(min)=(kq^2)/(5m_ev^2)](https://img.qammunity.org/2021/formulas/physics/college/69vuxa58gpdx6bdvx8sebxyypy4w4gb2id.png)
hence, the minimum distance between the two electrons is kq^2/5mv^2