Answer:
a) If the reaction has a large negative ΔGo value, the reaction must reach equilibrium at a small extent of reaction value
d) ΔGo and ΔG have the same magnitude, they just have opposite signs.
Step-by-step explanation:
The fraction of the total heat energy if a system that does useful work is known as Gibb's free energy (G) and the change from the initial to final state is designated by
 . It is observed that the values of
. It is observed that the values of
 changes with experimental conditions such as temperature , pressure , concentration etc.
changes with experimental conditions such as temperature , pressure , concentration etc.
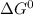 is the standard free energy change which is a balance of two natural tendencies of any system.
is the standard free energy change which is a balance of two natural tendencies of any system.
- Minimization of potential energy or enthalpic factor
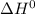
Maximization of disorderliness or entropic factor

Mathematically;
 =
=
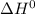 -
-

Thus; from above mentioned, the statements that are true about ΔG⁰ and ΔG are:
ΔG⁰ and ΔG can have different values, they don't even have to have the same sign
For a reaction that reaches equilibrium, the minimum value of free energy must be at the equilibrium point
If ΔG⁰ , measured at an extent of reaction = 0.5, is positive, the sign for ΔG when the extent of reaction = 0.80 is also positive.
while the false statements include:
a) If the reaction has a large negative ΔG⁰ value, the reaction must reach equilibrium at a small extent of reaction value
d) ΔG⁰ and ΔG have the same magnitude, they just have opposite signs.