Answer: Thus pOH has value of 8.19
Step-by-step explanation:
pH or pOH is the measure of acidity or alkalinity of a solution.
pOH is calculated by taking negative logarithm of hydroxide ion concentration.
![pOH=-\log [OH^-]](https://img.qammunity.org/2021/formulas/chemistry/college/hdm1ob4dj6mx2sy3kobrrj91lzbh3927bk.png)
Putting in the values:
![pOH=-\log[6.46* 10^(-9)]](https://img.qammunity.org/2021/formulas/chemistry/middle-school/zpd8efffo92xgm7rbl6sttj59x34appm7t.png)
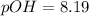
Thus pOH has value of 8.19