Answer:
200.87 g
Step-by-step explanation:
In order to calculate the mass of ice that could be melted by using 67,000 J of energy, we will use the following expression.
Q = ΔH°f × m
where,
- Q: heat required (67,000 J)
- ΔH°f: enthalpy of fusion of water (333.55 J/g)
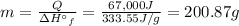
67,000 J of energy can be used to melt 200.87 grams of ice.