Answer:
Pressure of sulfur dioxide is 360mmHg
Step-by-step explanation:
The total pressure of a gas mixture is defined as the sum of the pressure of each gas in the mixture.
In the mixture of the problem, there are CO, CO₂ and SO₂ as gases. Total pressure is 1465mmHg, pressure of CO is 234mmHg and pressure of CO₂ is 871mmHg, it is possible to wirte:
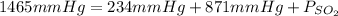
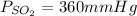
Thus, pressure of sulfur dioxide is 360mmHg.