Answer: 375 g
Step-by-step explanation:
Formula used :
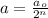
where,
a = amount of reactant left after n-half lives = ?
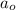 = Initial amount of the reactant = 100 g
= Initial amount of the reactant = 100 g
n = number of half lives = 4
Putting values in above equation, we get:
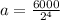
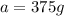
Therefore, the amount of carbon-14 remains at the end of four half-lives is 375 g