Answer:
There are 0.05 moles in 3.01*10²² atoms of chromium
Step-by-step explanation:
Mol is the amount of matter that contains Avogadro's number of unit particles or fundamental entities (these can be molecules, atoms, ions, electrons). So Avogadro's Number is the number of particles that make up a substance (usually atoms or molecules) and that can be found in the amount of one mole of that substance. Its value is 6,023 * 10²³ particles per mole.
Then a rule of three can be applied as follows: if 6.023 * 10²³ atoms are contained in 1 mole of substance, 3.01 * 10²² atoms in how many moles are they?
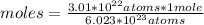
moles≅0.05
There are 0.05 moles in 3.01*10²² atoms of chromium