Answer:
option D
Step-by-step explanation:
Given,
V₁ = 285 L
P₁ = 530 mm Hg
Standard pressure
P₂ = 760 mm Hg
V₂ = ?
Using relation between pressure and volume
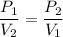
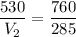
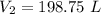
Hence, volume of the propane at standard pressure is equal to 198.8 L.
Hence, the correct answer is option D.