Answer:
1.839420926 x 10^8 m/s
Step-by-step explanation:
A form of De Broglie's equation can be derived from Einstein's formula
of E = mc^2
E = mc^2
E = hf
mc^2 = hf
We know f = v / λ
so mc^2 = hv / λ
That means that E = hv / λ
h, λ, c = constants
It now becomes easy to plug in our values:
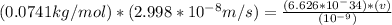
Solve for v
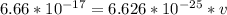
v = 100514804.7 mol*m/J*s
x Activation Energy = 183942092.6 m/s
This makes sense because the particles are photons and should be moving close to the speed of light.