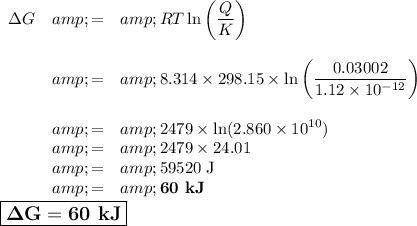Answer:
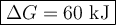
Step-by-step explanation:
Ag₂CrO₄ ⇌ 2Ag⁺ + CrO₄²⁻; Ksp = 1.12× 10⁻¹²
I/mol·L⁻¹: 0.191 0.823
To get ΔG under these conditions, we can use the equation
ΔG = ΔG° + RTlnQ = -RTlnK + RTlnQ = RTln[Q/K]
1. Calculate Q
![Q =\text{[Ag$^(+)$]$^(2)$[Cr$_(2)$O$_(4)^(2-)$]} = 0.191^(2)*0.823 = 0.03002](https://img.qammunity.org/2021/formulas/chemistry/college/d5eb1wimnl1s8ym2o2to17pfqddtmkbl75.png)
2. Calculate ΔG
T = (25.0 + 273.15) K = 298.15 K