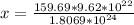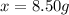Answer:
The grams of rust present in the bicycle frame is
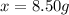
Step-by-step explanation:
From the question we are told that
The number of oxygen atom contained is
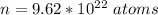
The molar mass of the compound is
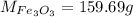
At standard temperature and pressure the number of oxygen atom in one mole of iron(III) oxide is mathematically evaluated as
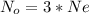
Where Ne is the avogadro's constant with a value

So
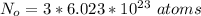
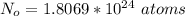
So
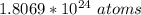 is contained in
is contained in
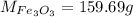
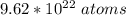 is contained in x
is contained in x
So