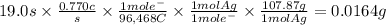Answer:
0.0164 g
Step-by-step explanation:
Let's consider the reduction of silver (I) to silver that occurs in the cathode during the electroplating.
Ag⁺(aq) + 1 e⁻ → Ag(s)
We can establish the following relations.
- 1 A = 1 C/s
- The charge of 1 mole of electrons is 96,468 C (Faraday's constant)
- 1 mole of Ag(s) is deposited when 1 mole of electrons circulate.
- The molar mass of silver is 107.87 g/mol
The mass of silver deposited when a current of 0.770 A circulates during 19.0 seconds is: