Answer:
A.) Balanced equation for the reaction:
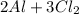 →
→
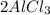
5.3 mol Al ×
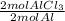 ×
×
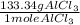 = 706.7g of
= 706.7g of
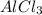 is produced as product
is produced as product
3 mol Cl ×
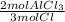 ×
×
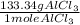 = 266.68g of
= 266.68g of
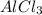 is produced as product
is produced as product
B.) The reactant that produces the list amount of product is the limiting reactant. Hence, Cl2 is limiting, while Al is in excess.
C.) moles of product formed is 2 moles.
D.) No of moles of the remaining excess reactant:
1 mole of CL - 3/2 mol of Al
3 mol of Cl - 3 ×
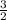 = 4.5 moles of Al.
= 4.5 moles of Al.
5.3 - 4.5 mole = 0.8 mol of Al remains.